Available since June 2020
Prof. Coenraad Hendricksen (DCVMN Consultant)
This e-training reviews principles, policies, measures, and practices to be taken to ensure good sanitation during manufacturing. These general considerations ensure the good functioning of the manufacturing facility.
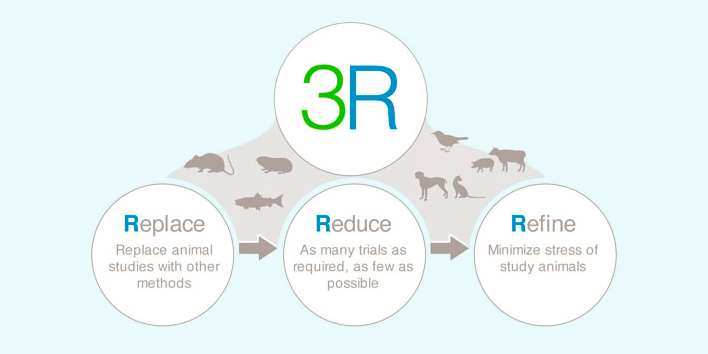
This tutorial will look at alternate methods and strategies to Refine, Reduce or Replace the use of animals (the Three R’s) in vaccine development and quality control. In addition, aspects of validation, acceptance, implementation, drivers and barriers are discussed.
- Self-Paced
- EN